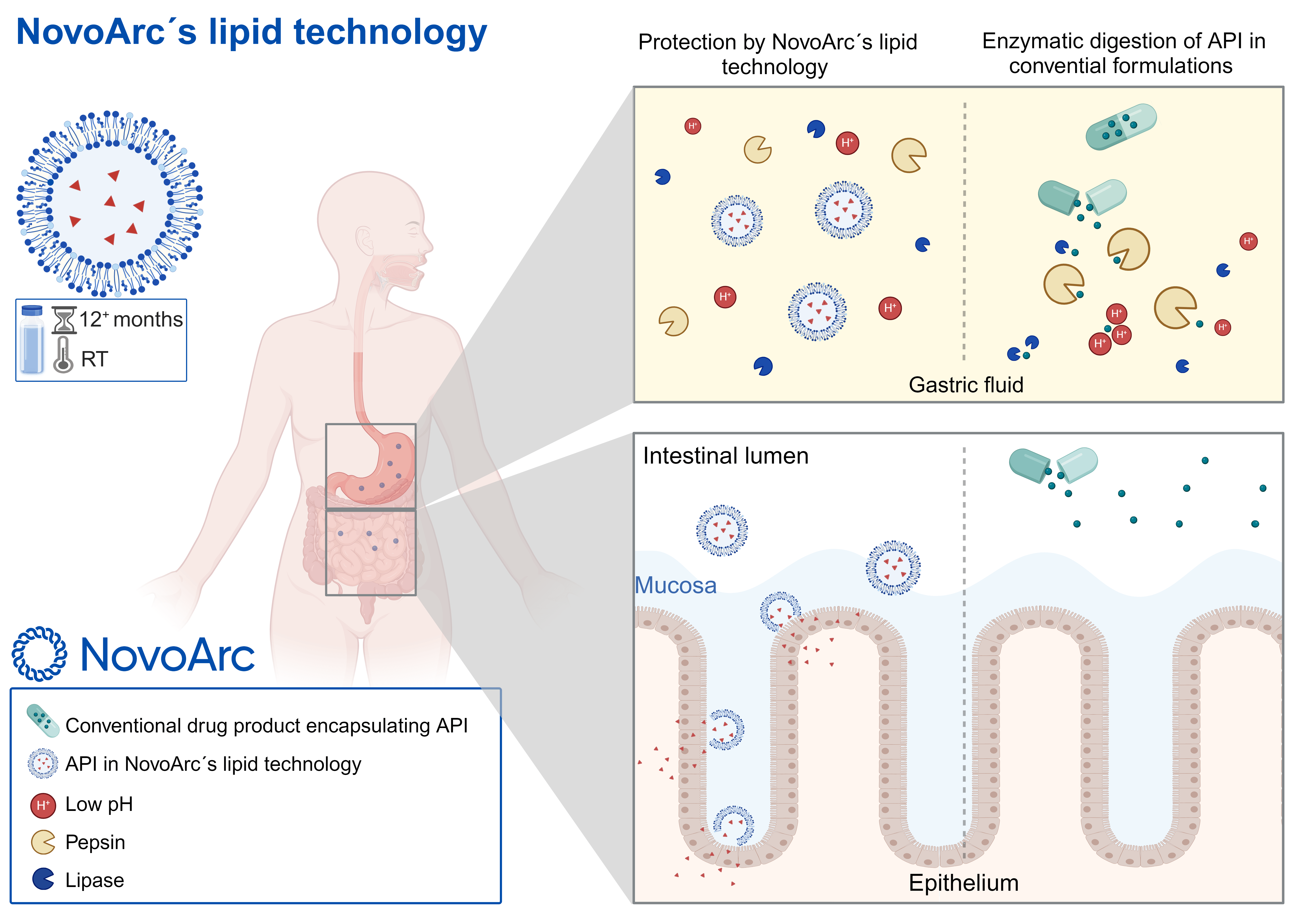
Conventional lipids, such as phospholipids, are sensitive to the harsh conditions of the gastrointestinal (GI) tract. They offer limited protection to drug substances and often fail to ensure effective delivery. As a result, conventional liposomes and lipid nanoparticles are typically administered via parenteral routes.
In contrast, NovoArc’s proprietary lipids and lipid-based formulations offer several key advantages for oral drug delivery:
-
Protection against gastrointestinal hydrolysis, enzymatic lipolysis, and particle agglomeration
-
Extended shelf life at room temperature
-
Enhanced bioavailability following oral administration
Therefore, NovoArc lipids can be used to deliver a variety of drug substances directly to the intestine. They have been tested in animal studies, demonstrating high tolerability after oral administration.
These properties make NovoArc’s lipid technology a strong platform for enabling the oral delivery of sensitive or poorly bioavailable compounds.
Encapsulating small molecules in liposomes enables controlled and sustained release within the body, optimizing therapeutic outcomes. This approach enhances the solubility of hydrophobic drugs, thereby improving their bioavailability and overall efficacy.
Moreover, liposomal delivery systems can reduce systemic toxicity and enable targeted delivery to specific tissues or cells, effectively minimizing side effects and increasing patient safety.
- CASE STUDY CANADIBIOL
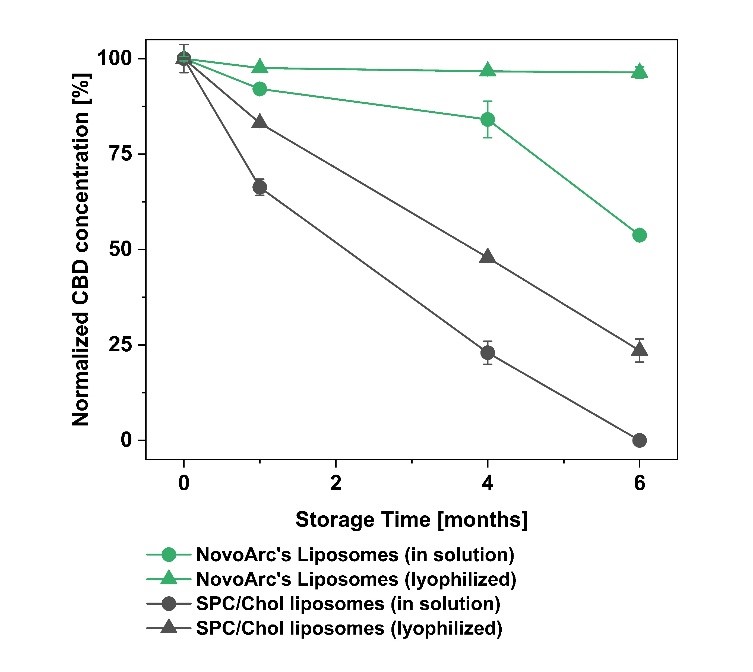
Tetraether lipids offer significant advantages for the encapsulation of cannabidiol (CBD), providing superior chemical, physical, and physiological stability compared to conventional liposomal formulations.
Long-term storage stability was demonstrated, with no degradation of CBD observed in NovoArc’s liposomes even after 6 months.
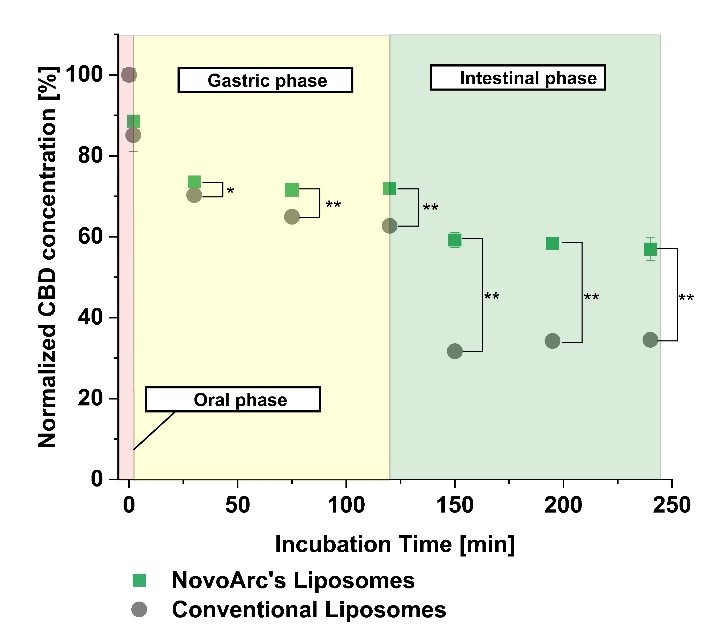
Under simulated gastrointestinal conditions, the formulation showed a 1.7-fold increase in stability, supporting its suitability for oral delivery.
In in vitro studies using colonic cells, a 6-fold higher uptake of NovoArc’s liposomes was observed, indicating enhanced intestinal absorption.
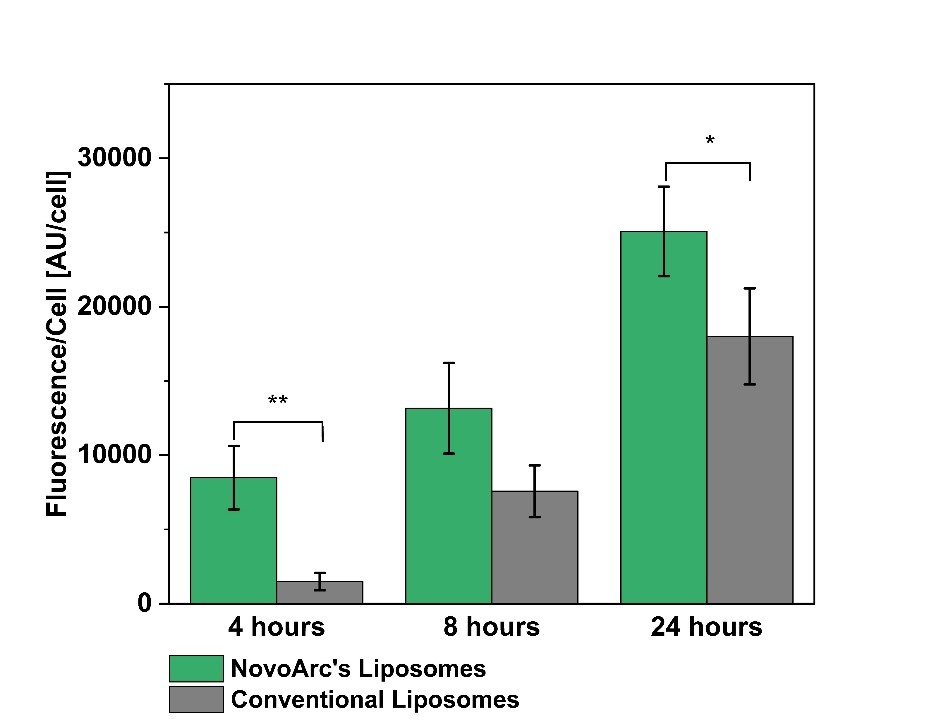
Encapsulating peptides and proteins in liposomes enriched with tetraether lipids offers multiple therapeutic advantages. This strategy protects sensitive biomolecules from enzymatic degradation, significantly enhancing their stability and prolonging their half-life within the gastrointestinal tract.
Beyond protection, these specialized liposomes facilitate targeted delivery by promoting cellular uptake and tissue penetration. Additionally, liposomal encapsulation can reduce immunogenicity and minimize adverse reactions, resulting in safer and more effective therapeutic outcomes.
- CASE STUDY INSULIN
Insulin, a therapeutic protein, is currently administered exclusively via subcutaneous injection, requiring a needle for each treatment. This mode of delivery can be inconvenient and uncomfortable, potentially reducing patient compliance.
Encapsulating insulin in highly stable lipid-based particles offers a promising alternative by enabling oral delivery. This approach has the potential to significantly improve patient convenience and adherence to therapy.
In a recent publication, NovoArc’s proprietary lipids demonstrated successful encapsulation of insulin, effectively protecting it from degradation in gastric fluids.

Figure: Cryo-TEM of lyophilized insulin containing liposomes
- CASE STUDY VANCOMYCIN
Vancomycin is a glycopeptide antibiotic widely used as a last-line treatment for infections caused by multi-resistant bacteria. Due to its very low oral bioavailability, vancomycin is currently administered exclusively via parenteral routes.
By encapsulating vancomycin in tetraether lipid-containing liposomes, we achieved a significant enhancement in oral absorption. In preclinical studies, this formulation resulted in an almost 9-fold increase in oral bioavailability, reaching 4.4%, compared to the negligible absorption seen with non-formulated vancomycin.
This demonstrates the potential of NovoArc’s liposomal technology to enable oral delivery of potent drugs that are otherwise limited to injection-based administration.
(Publication submitted)
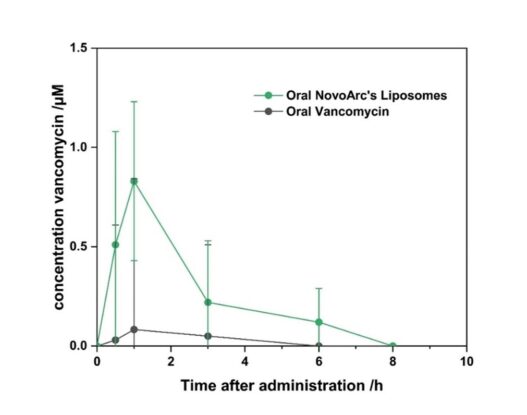
Figure: Vancomycin concentration in blood samples after oral administration.
In recent years, nucleotides such as plasmid DNA (pDNA), messenger RNA (mRNA), and microRNA have been the focus of intense research and therapeutic development. To achieve therapeutic efficacy, these sensitive molecules must be encapsulated in lipid nanoparticles (LNPs), which protect them from enzymatic degradation and facilitate their uptake into cells.
However, current LNP formulations are primarily designed for intramuscular administration and are not suitable for oral delivery, due to their limited stability in the harsh environment of the gastrointestinal tract.
By incorporating NovoArc’s proprietary tetraether lipids, it is possible to generate highly stable and resistant nanoparticles, offering the potential for improved oral bioavailability of nucleotide-based therapeutics.
- IN VITRO CASE STUDY MRNA
The native TELs (archaeal lipid extract) produced by NovoArc, as well as the purified GDGTs (glycerol dialkyl glycerol tetraether lipids), were incorporated into a commercial LNP formulation and resulted in a significant increase in particle uptake and transfection efficiency in Caco-2 cells (see figure below).
Our data clearly demonstrate the beneficial effects of tetraether lipids within LNP formulations and highlight their potential for enabling oral administration.

Figure: Increased particle uptake and increased expression of GDGT (glycerol dialkyl glycerol tetraether lipids) and ALE (archaeal lipid extract)-containing LNPs in C2C12 cells.
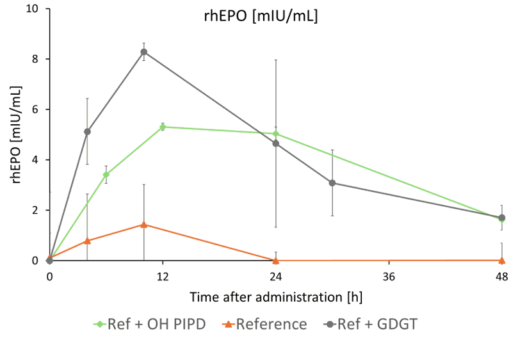
- IN VIVO CASE STUDY MRNA
The GDGT (native tetraether lipid) and OHPIPD-GDGT (a chemically modified, ionizable derivative) produced by NovoArc were used to develop an LNP formulation.
In an initial feasibility study, an oral in vivo pharmacokinetics (PK) study using mRNA encoding for human erythropoietin demonstrated a 1% bioavailability compared to parenteral delivery, representing an 11-fold increase compared to conventional LNPs.
(Publication in preparation)